Abstract
Introduction: Mature T-cell lymphomas (MTCLs) comprise a group of rare, heterogeneous entities with poor prognosis. Optimal use of autologous (auto) and allogeneic (allo) hematopoietic stem cell transplantation (HSCT) remains an area of active study (Marchi & O'Connor 2019). Prognostic scores help guide clinical decision making and are useful to assess baseline risk in retrospective studies. The Endothelial Activation and Stress Index (EASIX) has shown promise in predicting post-alloHSCT survival (Shouval et al. 2019, Luft et al. 2020). However, we have little data on the association between EASIX and post-HSCT survival specific to the MTCL population. Here, we assess the relationship between EASIX prior to conditioning chemotherapy (EASIX-pre) and post-HSCT survival.
Methods: Subjects with MTCL who underwent auto- or alloHSCT were identified from a Montefiore Medical Center database. Data were collected on the following by manual chart review: date of birth, sex, race, ethnicity, MTCL subtype, date of stem cell infusion (D0), stem cell source, conditioning intensity, and survival. To be included in the auto- or alloHSCT cohort, a subject's MTCL subtype had to have auto- or alloHSCT as a recommendation in the NCCN Guidelines Version 2.2022 (Horwitz et al. 2022). Conditioning intensity was determined by the Center for International Blood and Bone Marrow Transplant Research operational definition (Giralt et al. 2009). EASIX prior to conditioning (EASIX-pre) was calculated as (lactate dehydrogenase [U/L] x creatinine [mg/dL])/platelets [109 cells/L] at D-21. To estimate lab values at D-21 we used linear interpolation on data available from D-81 to D-9. Missing numerical variables were imputed using scikit-learn's IterativeImputer with the RandomForestRegressor estimator and default parameters, a method similar to missForest. The Nelson-Aalen estimate of the cumulative hazard and event status at last follow up were included in imputation (White & Royston 2009). Missing categorical variables were placed into an unknown category. Univariable Cox models were made for each variable. Variables whose Cox models had likelihood ratio test p < 0.20 were used for adjustment in multivariable models. Survival was measured from D0 to last follow up. Events included both death and discharge to hospice with unknown date of death. Data were analyzed using Python, lifelines, and sci-kit learn (Davidson-Pilon 2019, Pedregosa et al. 2011).
Results:
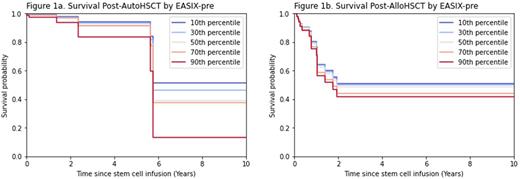
23 subjects underwent autoHSCT 9/19/2006-3/24/2021. Median follow up among censored subjects was 2.30 years. 9 subjects had peripheral T-cell lymphoma, NOS (PTCL, NOS), 7 had angioimmunoblastic T-cell lymphoma, 4 had ALK-negative anaplastic large cell lymphoma, and 3 had other subtypes. EASIX 10th, 50th, and 90th percentiles were 0.55, 1.51, and 3.68. The univariable model for age at D0 had likelihood ratio test p < 0.20 and was used for adjustment in the multivariable Cox model; other potential adjustment variables did not meet this criterion. EASIX-pre adjusted for age at D0 had HR 1.37 (95% CI 1.06-1.76, p = 0.0155, Fig. 1a).
32 subjects underwent alloHSCT 4/22/2008-4/5/2021. Median follow up among censored subjects was 3.36 years. 16 subjects had adult T-cell leukemia/lymphoma, 6 had hepatosplenic T-cell lymphoma, 4 had mycosis fungoides/Sezary syndrome, 4 had PTCL, NOS, and 2 had other subtypes. EASIX 10th, 50th, and 90th percentiles were 0.55, 1.16, and 2.82. The univariable models for age at D0, race, and conditioning intensity had p < 0.20 and were included for adjustment in a multivariable Cox model. In the multivariable model, EASIX-pre had HR 1.12 (95% CI 1.00-1.26, p = 0.0584, Fig. 1b).
Conclusion: In our MTCL cohort, at an alpha level of 0.05, EASIX-pre was independently associated with post-autoHSCT but not post-alloHSCT survival.
Disclosures
Shastri:NACE: Honoraria; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mantzaris:Kite Pharma: Honoraria. Sica:PER Physician's Education Review.: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Miragen: Consultancy, Honoraria; Kite Pharma: Research Funding; Bristol Myers Squibb Foundation: Research Funding; AstraZeneca: Honoraria, Research Funding; Curios: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.